Capabilities
Capabilities
Corrosion Analysis of Materials
Laboratory
National Renewable Energy Laboratory (NREL)Capability Expert
Judith Vidal, Todd Deutsch, James YoungClass
BenchmarkingCharacterization
Node Readiness Category
3: High-Temperature Electrolysis (HTE)1: Low-Temperature Electrolysis (LTE)
1: Photoelectrochemical (PEC)
Description
The analytical characterization of corrosion for advanced water splitting materials employs different tools depending upon the nature of the technology (photoelectrochemical, advanced electrolysis) under consideration so the following information is broken into the relevant subheadings. The corrosion analysis node at NREL has different readiness levels with respect to the different water splitting technologies. Corrosion analysis of photoelectrochemical materials is fully developed and is readily used at NREL. Corrosion analysis of low temperature electrolysis have been used for evaluating fuel cell bipolar plate materials is readily available. Also available is a scanning electrochemical microscope (SECM), an electroanalytical scanning probe tool that can be used to image substrate topography and local reactivity with high resolution. The SECM can be operated in several modes that make it a useful tool in a variety of applications. Some examples include:
- Determining values for kinetic rate constants
- Mapping and quantifying differences in H2 (or O2) production at surfaces
- Corrosion detection/monitoring
- Studying adsorbed surface species on an electrode.
Category 1: Photoelectrochemical Materials
This capability involves testing water-splitting semiconductors under simulated operational conditions and performing electrochemical and physical diagnostic testing. The capability has been funded by the FCTO for >5 years. Promising photocathodes and/or photoanodes can be tested under constant current or voltage bias, with an appropriate reference electrode, in a wide range of aqueous electrolytes. Diagnostic current-potential (J-V) or impedance-based (EIS) electrochemical testing can be performed at any point during the testing to evaluate degradation. Upon the completion of testing, the electrolyte can be analyzed by inductively coupled plasma mass spectrometry (ICP-MS) to quantify trace amounts (ppb) of relevant elements (Figure 1). ICP-MS can also be used to measure the loading of surface treatments, coatings, or co-catalysts by digesting the modified semiconductors in aqua regia and analyzing the resulting aqueous solutions for the elements of interest. Etching of tested electrodes can be evaluated using stylus or optical interference profilometry (Figure 2). If necessary, electrode surfaces can be analyzed by a suitable spectroscopic characterization tool (XPS, AES), scanning probe (AFM), scanning electron microscopy (SEM), or scanning transmission electron microscopy (STEM), under a different capability node, to better understand the chemical features and microstructure of degraded or stabilized electrode surfaces. A glove box is available for air-excluded sample testing, extraction and packaging prior to analysis.
Advanced Electrolytic (AE) Hydrogen Production. Low-and high-temperature electrolysis (including alternative chemistries):
Category 1: Low-Temperature Electrolysis Materials
NREL has extensive expertise on materials selection based on operating conditions and required working environment. To evaluate materials performance, and thus determine ways to optimize it, we perform a variety of materials characterization before and after material exposure to in-situ and ex-situ conditions. This characterization includes microstructural, chemical, mechanical, thermal, physical, and/or electrical evaluations. NREL has a variety of customized apparatuses for hydrogen production from biofuels, electrolysis for water splitting, photoelectrochemistry, and thermochemical processing.
Ex-situ materials degradation evaluations can be performed by exposing the materials to different environments (e.g., at selected temperatures under different controlled atmospheres), emulating the real conditions expected under real operation. Conventional long-term immersion techniques and electrochemical techniques are commonly employed to evaluate materials degradation. Electrochemical techniques are very fast responding and can determine mechanisms occurring at the material's surface. Changes associated with mass loss, chemical, and microstructural modifications can be determined. For water splitting, the most important material changes that we need to keep track of are those that can affect electrical, chemical, and mechanical properties.
Common electrochemical techniques used to evaluate materials performance in conductive liquid media such as aqueous solutions or molten salts are linear sweep voltammetry (LSV), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS), among others. EIS is a powerful technique used to determine fundamental mechanisms occurring near the surface and is used to characterize thin films, surface reactions and occurring physicochemical events.
Figure 1 shows the electrochemical setup used to carry out a study of the passivation behavior of the anodic sintered titanium (Ti) porous transfer layer (PTL) up to very high overpotential (3 V), with and without platinum coatings. The results in Figure 2 showed that anodic Pt-coated Ti PTL should be used in PEM electrolysis because no passivation at the surface of platinum coatings was formed to hinder electronic transfer.
For low-temperature advanced electrolysis of water, we have access to several electrochemical setups at low and elevated temperatures, thermogravimetric analyzer (TGA), gas chromatographs (GC), inductively coupled plasma with mass spectrometer (ICP-MS), X-ray diffractometer (XRD); several optical and electron microscopes (TEM, SEM), among other state-of-the-art characterization tools.
Category 3: High-Temperature Electrolysis Materials
Challenges associated with next-generation membranes, that need high conductivity and be resistant to corrosive electrolytes at high temperatures, can be addressed using current expertise and infrastructure at NREL. NREL has dedicated laboratory facilities for the evaluation of material systems under extreme conditions to help inform materials selection. Corrosion evaluations can be performed at selected temperatures under controlled atmospheres using long-term corrosion tests and electrochemical corrosion evaluations. Electrochemical impedance spectroscopy (EIS) is a powerful technique used to determine fundamental corrosion mechanism and is used to characterize thin films, surface reactions and physicochemical events occurring at the material’s interface. Additional electrochemical techniques are available to perform the complete corrosion evaluation of materials. Same characterization techniques as explained before are utilized here to understand corrosion mechanisms and mitigation effectiveness.
Chemical degradation of materials can be assessed under controlled conditions, including temperature, pressure, atmosphere (vacuum, inert, non-reactive, and reactive), gas flow control, fluids (organic and inorganic) and material chemistry fluids (organic and inorganic), time of exposure, thermal cycling, and rates of heating/cooling rates (up to ~100°C/min), among others.
Novel materials can be synthesized using electrodeposition of compounds dissolved into electrolytes. The morphology of the product can be controlled by controlling the temperature, voltage and current density of the system. Several instruments are also available to evaluate thermo-physical properties. Differential scanning calorimeters (DSCs), thermogravimetric analyzers (TGAs) with mass spectroscopy can be utilized under different atmospheres to determine chemical and thermal behavior of systems. High temperature wettability of surfaces with different fluids can be determined up to 850°C and rheological properties can be evaluated up to 550°C.
Capability Bounds
Laboratory scale for materials (organic and inorganic) characterization.
Photoelectrochemical
Electrode sizes are generally limited by the ability to uniformly illuminate their entire surfaces during laboratory testing (2x2 cm). Custom cells may be required to accommodate various electrode geometries.
Unique Aspects
Photoelectrochemical Materials
The collection of physical and chemical diagnostic tools coupled with long-standing photoelectrochemical expertise makes this node unique to NREL.
Low-Temperature Electrolysis Materials
Expertise and instruments are available to perform low-temperature electrolysis and chemical degradation tests at low temperature. High-volume manufacturing has been addressed by developing metal bipolar plates and coatings that are low-cost, lightweight, corrosion-resistant, gas impermeable, and amenable to mass manufacturing. The same approach will be used in HydroGEN to develop advanced materials for water splitting. NREL has developed the experimental testing protocol for corrosion measurements under aggressive polymer electrolyte membrane fuel cell (PEMFC) operational conditions and for measuring contact resistance. NREL is also unique in having a long experience in conducting oxides, and their high-speed application, because of their use in various types of solar cells.
High-Temperature Electrolysis Materials
NREL has wide-ranging experience in characterization of organic and inorganic materials applied to high-temperature renewable energy systems.
Under harsh conditions anticipated for high-temperature and/or high-solar intensity processes representative of applications for solar hydrogen production, the material/environment interface creates physical and chemical changes that must be monitored and quantified. Finding the right material for a given application is a product of selecting the cost-efficient combination of processing, structure, property and performance. Challenges in Hydrogen production resulting from high temperature, thermal cycling, delamination of material interfaces due to bubble formation, and operation in oxygen rich environments can be addressed and mitigated. The understanding of thermodynamic and kinetic aspects of the material interactions helps develop the mitigation approaches and material’s selection required. The characterization of materials is necessary to understand their behavior within each environment.
NREL has the expertise (staff and capabilities) necessary to identify and characterize advance materials for HydroGEN applications. This expertise is related with high- and low- temperature degradation, developing of coatings or processes to protect the surfaces, selection of advanced joining techniques, etc. Dr. Judith Vidal is the Building Energy Science Manager and the Thermal & Materials Research Team Lead of the CSP Thermal Sciences Group from NREL and is also a Joint Faculty at the Colorado School of Mines (CSM). She has more than 30 years of experience in chemical processing and chemical degradation of materials; characterization and enhancement of thermophysical properties of molten salts; and thermochemical modeling of advanced material systems. Dr. Vidal has extensive expertise in aqueous electrolytes (acid and basic), molten salts and high-temperature systems with several publications in peer-reviewed high-impact journals such as Nature, Material Degradation, Solar Energy Materials, and Solar Cells. Dr. Vidal co-authored the Gen3 CSP Roadmap and was the chair/leader of the molten salt technologies pathway in that document.
NREL has the unique capability to perform advanced electrochemical techniques to accelerate and study material degradations. We are considered experts in molten salt corrosion evaluations in controlled atmospheres at high temperatures (550 to 850°C). Surface mitigation approaches have been tested at NREL and several peer-review papers have been published. We have determined that surface treatments can mitigate corrosion in molten salts opened to air.
Material issues are always related to structure/property/synthesis of the materials. The characterization of materials in each step is key to understand the behavior of them in each environment. Our staff has some U.S. patents for electrodeposition of materials that will benefit the design and manufacturing of advanced AWS materials.
Availability
Photoelectrochemical Materials
Long-term durability testing is limited by availability of light sources that can vary based on demand. We have 7-8 durability testing stations that include potentiostats and light sources, permitting a relatively high throughput. A scientist unskilled in this area could gain moderate proficiency with a few days of training, and the number of redundant setups allows this capability to serve as a user facility. ICP-MS is typically batch processed once a suitable sample set (10-60) of electrolytes is accumulated. The ESIF Site Operations has dedicated personnel to maintain and provide support for ICP-MS.
Low- and High-Temperature Electrolysis Materials
The instruments are located in the user facility Energy System Integration Facility (ESIF) and hence available for internal research activities as well as for external partners and stakeholders. Instruments are annually calibrated, and maintenance is current. Users must be trained to use the instruments. Besides the electrochemical cells and apparatus, characterization tools are in ESIF and NREL site for direct access.
Benefit
Photoelectrochemical Materials
This capability can identify the native stability of potential water splitting semiconductors and benchmark the effectiveness of protective surface modifications.
Low- and High-Temperature Electrolysis Materials
Advanced materials characterization is key to understand synthesis path, materials behavior, chemical and mechanical degradation. This is directly related with materials selection for Hydrogen production such as containment materials and seals for high-temperature solid oxide electrolysis (SOEC). By using electrochemical techniques, corrosion of materials can be determined, understood, controlled and mitigated. Hydrogen cells are subject to corrosion and they should be evaluated to determine the best and cost-effective corrosion mitigation approaches that can be used. Long term corrosion tests can later be used to determine if changes in the corrosion mechanisms occur with time. Hydrogen production areas such advanced electrolysis (AE), photoelectrochemical (PEC) and low temperature part of a hybrid cycle, will directly benefit from proper advanced materials selection, characterization and synthesis.
Images
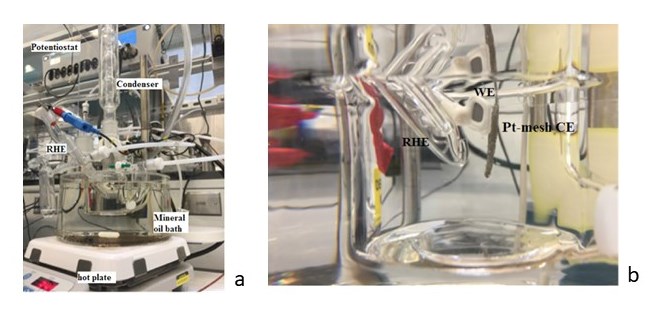
Figure 1. (a) Electrochemical cell for ex-situ corrosion analysis for low-temperature water splitting technologies, (b) Three-electrode arrangement for electrochemical corrosion evaluations (RE: RHE; CE: Pt-mesh; WE/S: PTL-Ti or PTL-PtTi).

Figure 2: Linear sweep voltammetry (LSV) for bare and platinized titanium PTL performed at 65 °C in 1M H2SO4. LSV was performed at 1 mV/s from open circuit potential to 3.3 V vs RHE.

Photoelectrochemical Materials: Post-durability analysis of p-GaAs photocathode using a) stereomicroscopy, b) optical profilometry, c) stylus profilometry, d) SEM, e) ICP-MS of electrolyte, f) XPS, and g) STEM of PtRu nanoparticle catalyst modified p-GaInP2.

Low- and high-temperature electrolysis materials: a) electrochemical cells, b) long-term immersion cells under controlled temperature, c) water and oil baths to control temperature, d) potentiostats and frequency response analyzer, e) open to room atmosphere and controlled atmosphere furnaces, f) metallographic sample preparation stations for surface and cross-section analyses, g) FESEM, h) ICP-MS, i) XRD, j) XPS and k) four-points probe.
References
Photoelectrochemical Materials
- Fatima Toor, Todd G. Deutsch, Joel W. Pankow, William Nemeth, Arthur J. Nozik and Howard M. Branz,Novel Micropixelation Strategy to Stabilize Semiconductor Photoelectrodes for Solar Water Splitting Systems, J Phys Chem C, 116(2012) 19262-19267.
- James L. Young, K. Xerxes Steirer, Michael J. Dzara, John A. Turner, and Todd G. Deutsch Remarkable stability of unmodified GaAs photocathodes during hydrogen evolution in acidic electrolyte, , J. Mater. Chem. A, 4(2016)2831 - 2836.
Low- and High-Temperature Electrolysis Materials
- Judith C. Gomez-Vidal, Corrosion resistance of MCrAlX coatings in a molten chloride for thermal storage in concentrating solar power applications. Nature npj Materials Degradation, (2017) 1–7. doi:10.1038/s41529-017-0012-3.
- J.C. Gomez-Vidal, A.G. Fernandez, R. Tirawat, C. Turchi, and W. Huddleston, Corrosion resistance of alumina-forming alloys against molten chlorides for energy production. II: Electrochemical impedance spectroscopy under thermal cycling conditions. Solar Energy Materials and Solar Cells, 166 (2017) 234–245.
- J.C. Gomez-Vidal, A.G. Fernandez, R. Tirawat, C. Turchi, and W. Huddleston, Corrosion resistance of alumina-forming alloys against molten chlorides for energy production. I: Pre-oxidation treatment and isothermal corrosion tests. Solar Energy Materials and Solar Cells, 166 (2017) 222–233. J.C. Gomez-Vidal, J. Noel, and J. Weber. Corrosion evaluation of alloys and MCrAlX coatings in molten carbonates for thermal solar applications. Solar Energy Materials & Solar Cells 157 (2016) 517–525. J.C. Gomez-Vidal and R. Tirawat, Corrosion of alloys in a chloride molten salt (NaCl‒LiCl) for solar thermal technologies. Solar Energy Materials & Solar Cells, 157 (2016) 234–244.
- J.C. Gomez-Vidal, E. Morton, Castable Cements to prevent corrosion of metals in molten salts. Solar Energy Materials & Solar Cells, 153 (2016) 44–51.
- M. P. Brady, H. Wang and J. A. Turner, Surface Modified Stainless Steels for PEM Fuel Cell Bipolar Plates, US7247403 B2, Jul 24, 2007.
- Heli Wang and John A. Turner. Electrochemical nitridation of a stainless steel for PEMFC bipolar plates, International Journal of Hydrogen Energy 36 (2011) 13008-13013.
- Heli Wang and John A. Turner. Austenitic stainless steels in high temperature phosphoric acid, Journal of Power Sources 180 (2008) 803–807.
- Heli Wang and John A. Turner: SnO2: F Coated Ferritic Stainless Steels for PEM Fuel Cell Bipolar Plates, Journal of Power Sources, 170 (2007) 387-394.
- Heli Wang, Glen Teeter and John A. Turner. Investigation of a Duplex Stainless Steel as Polymer Electrolyte Membrane Fuel Cell Bipolar Plate Material, Journal of the Electrochemical Society, 152 (3) (2005) B99-B104.
- Heli Wang, Michael P. Brady, K. L. More, H. M. Meyer III and John A. Turner. Thermally Nitrided Stainless Steels for Polymer Electrolyte Membrane Fuel Cell Bipolar Plates, Part 2: Beneficial Modification of Passive Layer on AISI446, Journal of Power Sources 138 (2004) 79-85.
- Heli Wang, Mary Ann Sweikart, John A. Turner. Stainless Steel as Bipolar Plate Material for Polymer Electrolyte Membrane Fuel Cells, Journal of Power Sources 115 (2003)243-251.
- C Liu, M Carmo, G Bender, A Everwand, T Lickert, JL Young, T Smolinka, D Stolten, W Lehnert. Performance Enhancement of PEM Electrolyzers through Iridium-coated Titanium Porous Transport Layers. Electrochemistry Communications.
