Capabilities
Capabilities
First Principles Materials Theory for Advanced Water Splitting Pathways
Laboratory
National Renewable Energy Laboratory (NREL)Capability Expert
Stephan LanyClass
Computational Tools and ModelingNode Readiness Category
3: High-Temperature Electrolysis (HTE)2: Low-Temperature Electrolysis (LTE)
1: Photoelectrochemical (PEC)
1: Solar Thermochemical (STCH)
Description
First principles materials theory (FPMT) supports the mission of HydroGen by providing atomic and electronic structure modeling capabilities, utilizing density functional theory (DFT) and many-body GW methods, as well as parameterized Hamiltonians bridging the gap between. We offer approaches, workflows, and expertise in supercell defect calculations [1], materials discovery and crystal structure predictions for both bulk and interface and surface phases [2], Monte-Carlo simulations of disordered systems [3], materials stability and convex hull analysis, electronic structure and optical properties [4], and electronic transport mechanisms [5]. The combination of DFT and beyond-DFT approaches allows predictions of accurate band offsets and ionization potentials. Addressing a wide range of materials properties and behavior enables comprehensive materials design strategies [6]. By combining first principles data with thermodynamic modeling [7], we predict advanced defect equilibria that simulate thermochemical redox processes at high temperatures [1].
The FPMT node currently supports projects in solar thermochemical hydrogen (STCH) production and provides capabilities that are also relevant for photoelectrochemical (PEC) hydrogen and electrolysis (LTE and HTE).
Capability Bounds
N/A
Unique Aspects
- Prediction of band gaps and optical properties for transition metal oxides
- Band-offsets, ionization potentials, electron affinities from beyond-DFT methods
- Atomic structure prediction from first principles for interfaces and surfaces
- Advanced defect equilibria for non-dilute interacting defects
- Carrier self-trapping and small-polaron transport
Availability
Utilization of DOE-EERE sponsored high performance computing (Eagle and Kestrel at NREL)
Benefit
The capability node provides access to important materials properties that are unknown for many materials of interest and are difficult to unambiguously determine experimentally. This capability will aid the selection of suitable materials and accelerate their optimization for water splitting.
Images

Prediction of band gaps and optical absorption spectra (blue) in MnZnO alloys for PEC water splitting [6], showing excellent agreement with experiment (red).
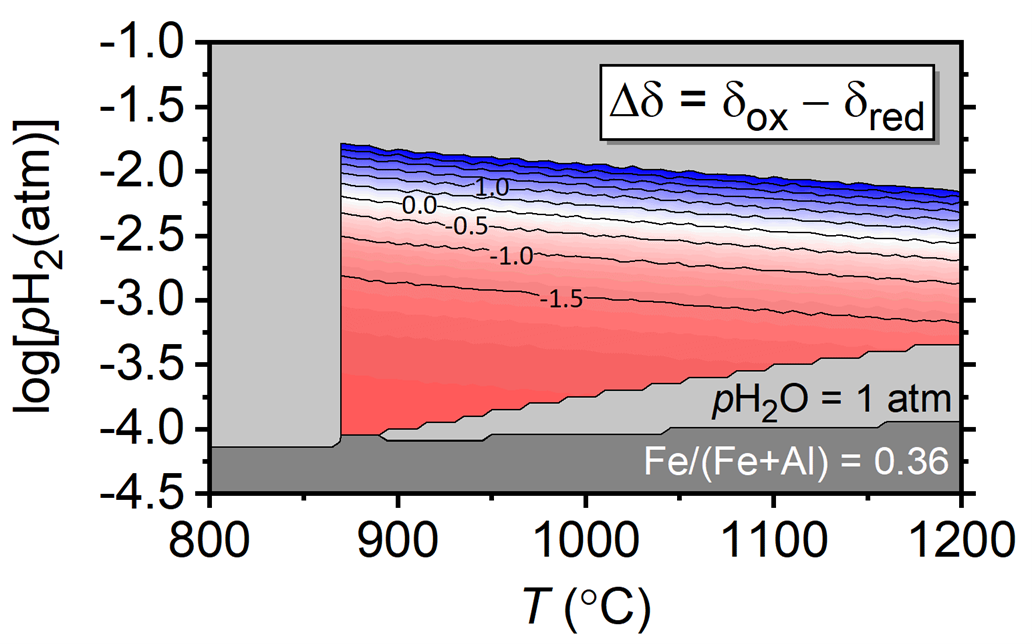
Modeled STCH hydrogen production capacity Dd in FeAl2O4-d as function of the temperature of the oxidation step and the H2 partial pressure [1].
References
[1] S.L. Millican, J.M. Clary, C.B. Musgrave, S. Lany, Redox defect thermochemistry of FeAl2O4 hercynite in water-splitting from first principles methods, Chem. Mater. 34, 519 (2022)
[2] A. Sharan, S. Lany, Computational Discovery of Stable and Metastable Ternary Oxynitrides, J. Phys. Chem. 154, 234706 (2021)
[3] J.J. Cordell, J. Pan, A.C. Tamboli, G.J. Tucker, and S. Lany, Probing configurational disorder in ZnGeN2 using cluster-based Monte Carlo, Phys. Rev. Mater. 5, 024604 (2021)
[4] S. Lany, Semiconducting transition metal oxides, J. Phys.: Cond. Matter 27, 283203 (2015)
[5] H. Peng, S. Lany, Semiconducting transition metal oxides based on d5 cations: Theory for MnO and Fe2O3, Phys. Rev. B (Rapid Comm.) 85, 201202(R) (2012)
[6] H. Peng, P. Ndione, D.S. Ginley, A. Zakutayev, S. Lany, Design of semiconducting tetrahedral Mn1-xZnxO alloys and their application to solar water splitting, Phys. Rev. X 5, 021016 (2015)
[7] S. Lany, The electronic entropy of charged defect formation and its impact on thermochemical redox cycles, J. Chem. Phys. 148, 071101 (2018)
