Capabilities
Capabilities
LAMMPS: Open-Source, High-Performance, and High-Fidelity Molecular Dynamics Code for Simulations of Chemical and Physical Processes of Materials
Laboratory
Sandia National Laboratories (SNL)Capability Expert
Reese Jones, Xiaowang Zhou, Steven Plimpton, Aidan ThompsonClass
Computational Tools and ModelingNode Readiness Category
2: High-Temperature Electrolysis (HTE)1: Low-Temperature Electrolysis (LTE)
2: Photoelectrochemical (PEC)
2: Solar Thermochemical (STCH)
2: Hybrid Thermochemical (HT)
Description
LAMMPS is a classical molecular dynamics (MD) code widely used within the materials science community. It has interatomic potentials for solid-state materials (metals, semiconductors), soft matter (biomolecules, polymers) and coarse-grained or mesoscopic systems. It can be used to model atoms or, more generically, as a parallel particle simulator at the atomic, meso, or continuum scale. It runs on a single processor or on large clusters of multicore or GPU compute nodes. It combines spatial-decomposition of the simulation domain over the nodes and thread-based parallelization within each node to achieve both high parallel efficiency and single-node performance. The code is designed to be easy to modify or extend with new functionality and is distributed as open source under the terms of the GPL. In addition to the core code, we have also developed the Atom-to-Continuum (ATC) module that enables coupled MD-finite element method (FEM) simulation and on-the-fly estimation of continuum fields.
Specific uses relevant to HydroGen-AWSM include:
- Calculation of energy barriers for water to dissociate on a metal surface
- Calculation of diffusion coefficients of hydrogen in materials
- Calculation of energies and structures of defects within solid materials (e.g. dislocations, grain boundaries, interfaces)
- Modeling the transition from the close-packed ions near electrodes to the diffuse bulk region in real double layers
- Modeling of extrinsic energy sources interacting with the intrinsic physics of MD, such as laser illumination exciting electronic states that ultimately convert to lattice heating
Capability Bounds
LAMMPS is designed to run on a variety of platforms from single processor, small machines to massively parallel computers, including advanced many-core and CPU/GPU architectures. Classical molecular dynamics is generally limited to small time scales but can resolve many equilibrium and transport processes over relevant length-scales and can leverage techniques such as parallel replica dynamics to extend accessible timescales.
Unique Aspects
LAMMPS is a true community code with tremendous and varied capability developed over 20 years by more than a hundred contributors. LAMMPS provides an unrivalled range of different interatomic potentials for oxide systems, ranging from simple pair potentials to polarizable core-shell models and complex manybody potentials such as COMB and ReaXFF, which enables modeling of a variety of fluid phase reactive chemistries. LAMMPS also provides interfaces to other atomistic modeling software such as KIM, QUIP and Quantum ESPRESSO. These capabilities allow users to quickly try out different models on a particular material and test structure, without having to repackage a lot of the input data.
Sandia’s unique bond order potentials enable high fidelity MD simulations of a variety of materials including metal alloys and compounds, semiconductor compounds, and oxides. Recently demonstrated MD simulations capture not only physical processes (e.g., tensile tests), but also chemical reactions (e.g., absorption of hydrogen from H2 gas sources by a metal surface). The ATC module provides the ability to simulate large, multiphysics processes with atomic detail in regions of interest and realistic interactions with the surrounding environment. This capability has been demonstrated on a number of technologically relevant applications ranging from phononic and electronic thermal transport in solid and fluid systems to ionic transport in fluids and coupled drift-diffusion in electrolytes interacting with electrodes.
Availability
Our methods are published with the LAMMPS open source code with a GPL license; see the LAMMPS Molecular Dynamics Simulator.
Benefit
Modeling atomistic interactions of complex material systems (such as corrosion or surface oxygen exchange) will generate a deeper, more fundamental understanding of water-splitting material behavior. This information can be used to derive and test novel material formulations leading to discovery, or analyze failure modes in materials and test mitigation strategies.
Images

Figure 1. MD simulation of oxidation of an aluminum layer on an Fe-Ni-Co substrate, showing the formation of a pinhole.
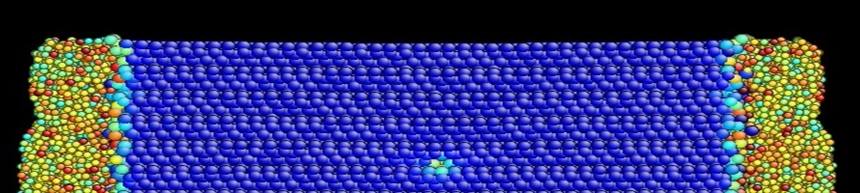
Figure 2. MD simulation of interaction between a dislocation in bulk Si and surface SiO2, showing dislocation motion under the stress created by the surface oxides.

Figure 3. MD simulation of graphene growth on Cu, showing the self-assembly of the graphene structure from random carbon adatoms.

Figure 4. MD simulation of 2H→H2 chemical reaction, showing that hydrogen atoms correctly form the H2 gas at room temperature.

Figure 5. Fluid electrolyte interacting dynamically with a solid electrode. Mesh shown is used to represent smooth fields such as the electric potential and concentration gradient.

Figure 6. Spatial resolution of ionic conductivity in a channel via partitioned Green-Kubo method.

Fig. 7 Surface coverage of H2O, OH−, and N+ in the ionomer for q = −0.10e/atom (top-down and side views of instantaneous configurations). (a) Bare Ni, (b) Ni covered by a monolayer of NiO, and (c) Ni with an ≈50% partial NiO coverage quarter disk monolayer centered on the lower left corner of the top view. Atom color map, H: white, O: red, N+: blue, Ni: gray, O in NiO: orange.
References
S. Plimpton, Fast Parallel Algorithms for Short-Range Molecular Dynamics, J Comp Phys, 117, 1-19 (1995)
X. W. Zhou, H. N. G. Wadley, J. S. Filhol, and M. N. Neurock, “Modified charge transfer-embedded atom method potential for metal metal oxide systems”, Phys. Rev. B, 69, 035402 (2004).
X. W. Zhou, H. N. G. Wadley, and D. X. Wang, “Transient hole formation during the growth of thin metal oxide layers”, Comp. Mater. Sci., 39, 794 (2007).
X. W. Zhou, D. K. Ward, and M. E. Foster, “An Analytical Bond-Order Potential for Carbon”, J. Comp. Chem., 36, 1719 (2015).
X. W. Zhou, D. K. Ward, M. Foster, and J. A. Zimmerman, “An analytical bond-order potential for the copper-hydrogen binary system”, J. Mater. Sci., 50, 2859 (2015).
R. E. Jones, D. K. Ward, J. A. Templeton, “Spatial resolution of the electrical conductance of ionic fluids using a Green-Kubo method”, J. Chem. Phys., 141, 184110, 2014
F. Rizzi, R. E. Jones, B. J. Debusschere, O. M. Knio. “Uncertainty quantification in MD simulations of concentration driven ionic flow through a silica nanopore. Part I: sensitivity to physical parameters of the pore.” J. Chem. Phys., 138:194104, 2013
F. Rizzi, R. E. Jones, B. J. Debusschere, O. M. Knio. “Uncertainty quantification in MD simulations of concentration driven ionic flow through a silica nanopore. Part II: uncertain potential parameters.” J. Chem. Phys., 138:194105, 2013
M. Salloum, K. Sargsyan, R. Jones, B. Debusschere, H. N. Najm, and H. Adalsteinsson. “A Stochastic Multiscale Coupling Scheme to Account for Sampling Noise in Atomistic-to-Continuum Simulations.” Multiscale Model. Simul. 550–584, 2012.
J. A. Templeton, R. E. Jones, J. W. Lee, J. A. Zimmerman, and B. M. Wong. “A long-range electric field solver for molecular dynamics based on atomistic-to-continuum modeling.” J. Chem. Theo. Comp., 7(6):1736–1749, 2011
RE Jones, WC Tucker, MJL Mills, S Mukerjee, “Insight into hydrogen production through molecular simulation of an electrode-ionomer electrolyte system” Journal of Chemical Physics 151, 034702 (2019)
RE Jones, WC Tucker, JM Rimsza, LJ Criscenti, “Atomic-scale interaction of a crack and an infiltrating fluid.” Chemical Physics Letters, 1:100005 (2018)
Rimsza, Jessica M., Reese E. Jones, and Louise J. Criscenti. “Interaction of NaOH solutions with silica surfaces.” Journal of colloid and interface science 516 (2018)
Jones, R. E., D. K. Ward, F. S. Gittleson, and M. E. Foster. “Assessing electrolyte transport properties with molecular dynamics.” Journal of The Electrochemical Society 164, no. 6 (2017)
Jones, Reese E., and Donald K. Ward. “Influence of defects on the thermal conductivity of compressed LiF.” Physical Review B, 97 no. 5 (2018)
