Capabilities
Capabilities
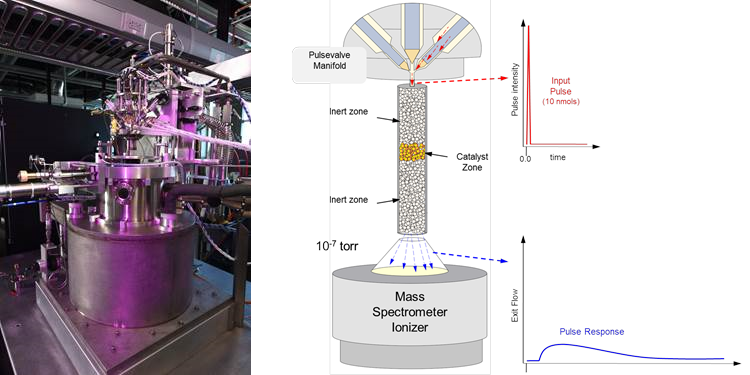
Temporal Analysis of Products (TAP) Reactor System
Laboratory
Idaho National Laboratory (INL)Capability Expert
Rebecca FushimiClass
CharacterizationMaterial Synthesis
Node Readiness Category
1: High-Temperature Electrolysis (HTE)2: Solar Thermochemical (STCH)
Description
The TAP Reactor system is a transient pulse response technique for collecting detailed kinetic information on complex, industrial catalysts and chemically active surfaces. By removing the material from the complexity of the operating environment the intrinsic kinetic function of the material in controlling chemical reactions can be more clearly assessed. In a TAP pulse response experiment, an ultralow intensity pulse (10 nmol) is injected into the microreactor under vacuum conditions. Transport in the reactor is well-defined Knudsen diffusion and any additional time delays of exiting molecules are attributed to a kinetic process. Theoretical techniques are well-developed for extracting quantitative kinetic data. Probe molecules can be selected to distinguish certain bond breaking/formation reactions. The level of kinetic rigor is similar to that found in molecular beam scattering experiments; but with TAP, complex materials can be assessed to the same extent directly from an operating environment. Relevant to high temperature water splitting, TAP can be used to support the development of complex catalysts applicable to heterogeneous reactions, or to measure surface adsorption and reaction behavior of SOFCs and STCH materials Assessing how material composition and processing pretreatment influence molecule interactions can shed light on how the structure/composition/kinetics function at a fundamental level. With a long pulse series one can incrementally track changes in composition (oxidation/reduction/deactivation) and then gauge the impact on kinetics.
Capability Bounds
Materials are typically studied in the powder or particular form including wires, foils gauzes, monoliths and single crystals can be accommodated as well. Probe molecules must be delivered in the vapor phase. Reactor temperature is currently < 1200 °C. Kinetic testing is carried out under high vacuum conditions but qualitative experiments can be extended to high pressure transient experiments (up to 2 atm).
Unique Aspects
The TAP Reactor System at INL is unique in the DOE complex. INL retains two of the three TAP Reactor Systems in the United States (the third is in operation at Harvard).
Availability
The instrument presently supports numerous projects but the addition of a second unit in September 2016 will greatly expand the capacity to take on new work. Our group is developing a 'user facility' type arrangement to increase the accessibility of the equipment to nonprofit groups. In order to apply TAP to HTWS applications, some enhancements to the TAP instrument need to made to accommodate acidic gas mixtures, or to raise the operating temperature.
Benefit
The TAP reactor can be used to direct high-throughput testing with detailed kinetic information for comparing distinctive composition, synthesis and processing procedures. High-throughput testing provides a wide breadth of composition evaluation but only provides limited steady-state kinetic information about the slow reaction steps. TAP provides a greater depth of detailed, microkinetic information on complex materials that are not amenable to atomistic modeling. The TAP technique can be used to better understand the composition/kinetics relationship for hydrogen storage, water/hydrogen/oxygen surface kinetics, reforming processes and catalysts, PROX reactions, CO and S poisoning, etc.
References
General Review:
J. Perez-Ramirez, E.V. Kondratenko, Evolution, achievements, and perspectives of the TAP technique, Catal. Today, 121 (2007) 160-169.
S. Shekhtman, A. Goguet, R. Burch, C. Hardacre, N. Maguire, CO multipulse TAP studies of 2% Pt/CeO2 catalysts: Influence of catalyst pretreatment and temperature on the number of active sites observed, Journal of Catalysis 253 (2008) 303-311.
K. Morgan, K. Cole, A. Goguet, C. Hardacre, G. Hutchings, N. Maguire, S. Shekhtman, S. Taylor, TAP studies of CO oxidation over CuMnOx and Au/CuMnOx catalysts, Journal of Catalysis 276 (2010) 38-48.